Abstract
Background. Outcome of patients (pts) with R/R AL is dismal. While traditional precision medicine basket trials have assigned treatment based on targeted inhibitors for certain mutations, this approach has limited use for pts without such mutations or with co-occurring mutations. In contrast, we developed a clinical trial for which treatment was assigned based on molecular characteristics and functional drug testing. Patient cell samples were analyzed for 194 recurring mutations and by high throughput screening (HTS) for a custom panel of drugs and drug combinations to comprehensively select the best treatment for each individual patient. Here we report the final analysis and long-term outcomes of this individualized approach. (NCT02551718)
Methods. Eligibility criteria included diagnosis of AL, relapsed or refractory to at least 2 lines of treatment, ECOG <4 and adequate organ function.
Blood/bone marrow (BM) and/or fluid or tissue involved by leukemic blasts was subjected to cell enrichment using magnetic beads, then assayed for drug sensitivity using the CLIA approved Cancer Drug Sensitivity Test (CDST) performed at the University of Washington Quellos High Throughput Core (method described in Lee et al. Nat Commun 2018), mutations by MyAML®, RNAseq and single cell mutation analysis (MissionBio). The HTS included 170 drugs and drug combinations, both FDA-approved and investigational. Drug recommendations were provided by the study to the patient's physician based on the lowest IC50, percentile rankings compared to other tested patients, no history of failed prior use, and insurance authorization. The patient's physician made the final decision about treatment.
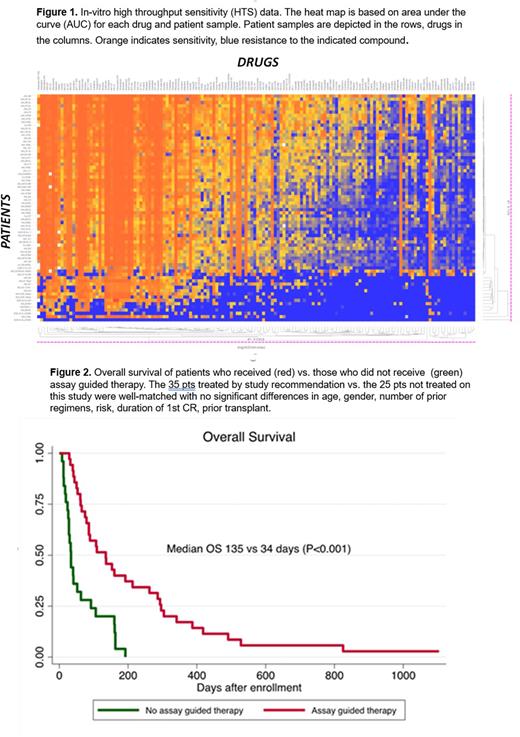
Results. 60 pts were enrolled: 51 acute myeloid leukemia (AML), 7 acute lymphoblastic leukemia (ALL), 1 undifferentiated, 1 mixed phenotype. The median age was 58 years (range 22-82). For AML pts, ELN 2017 was favorable, intermediate and adverse in 8 (16%), 14 (28%) and 28 (56%) pts, respectively. Median prior lines of therapy were 4 (range 1-8); 15 pts (25%) had primary induction failure and 23 pts (40%) had relapsed post allogeneic transplant, 10 in the first 100 days (d). For those who had relapsed disease, median duration of 1st CR was 6.8 months (range 0.5-52.6). Final HTS test results were obtained within 5 d (4-13), and each patient exhibited a unique pattern of drug response (Figure 1).
35 patients were treated with a single drug (N=12) or combination (2-4 agents; N=23) recommended by the trial. 25 of the patients did not complete treatment on this study because of transition to supportive care (6), treatment with other therapy (10), travel out of state to local care (4), participation on another trial (CAR-T cell-2, investigational agent-2), and pneumonia (1).
30 patients had at least 1 mutation for which targeted inhibitors [FLT3 (11), KIT (2), IDH1/2 (7), NRAS/KRAS (20), ABL1 (3), JAK2 (1)] were available, and seven patients received one. Additionally, 35 pts (4 had more than 1) had a mutation that could now be considered for investigational therapy, including TP53 (12), NPM1 (8), KMT2A rearrangement (4), WT1 (10), or spliceosome mutation (5).
Median follow up duration was 2.7 months (range 0.3-36.3). Of 27 pts for whom a post-treatment BM was performed, overall response rate was 33% including 2 complete remission (CR), 1 CR with incomplete count recovery (CRi) and 6 partial remissions (PRs). Of 22 pts with circulating blasts, 21 (95%) exhibited decline with chosen regimen, and 7 (31.8%) exhibited resolution. Median overall survival (OS) for those receiving assay guided therapy was 135 days (d) (95%CI 79-213), compared to 34 d (95%CI 26-63) for enrolled patients not treated on study with corresponding 100 day-OS of 57% (95%CI 39-72%) and 24% (95%CI 10-42), respectively (P<0.001) (Figure 2). Median survival post consent was 109 d (95%CI 79-341) for the subset of post-allogeneic transplant relapsed pts who then received assay guided therapy vs. 30 d (95%CI 8-92) for the those not treated on this trial (P 0.003). Assay guided therapy can decrease risk of death by 83% after adjusted by age, sex, type of leukemia, cytogenetic risk, number of lines of previous treatment (HR 0.17, 95%CI 0.08-0.35, P <0.001) for this group of pts with extensive prior therapy.
Conclusion. Genomic analysis and functional screening of enriched leukemia cells can comprehensively predict in vivo efficacy and individualize optimal treatment for patients with R/R AL.
Disclosures
Kongtim:CareDx, Inc.: Consultancy. Percival:Ascentage: Research Funding; Abbvie: Research Funding; Celgene/BMS: Research Funding; Biosight: Research Funding; Glycomimetics: Research Funding; Cardiff Oncology: Research Funding; Oscotec: Research Funding; Trillium: Research Funding; Pfizer: Research Funding; Telios: Research Funding. Oehler:Pfizer: Consultancy, Research Funding; Novartis: Consultancy; Blueprint Medicines: Consultancy; Bristol Myers Squibb: Consultancy. Cassaday:Vanda: Research Funding; Pepromene Bio: Membership on an entity's Board of Directors or advisory committees; Autolus: Membership on an entity's Board of Directors or advisory committees; Jazz: Consultancy; Servier: Research Funding; Merck: Research Funding; Kite, a Gilead Company: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Seagen: Current Employment, Current equity holder in private company, Other: Spouse employment with Seagen; stock or other ownership in Seagen.; Amgen: Consultancy, Research Funding. Halpern:Bayer Pharmaceuticals: Research Funding; Imago Biosciences: Research Funding; Tolero Pharmaceuticals: Research Funding; Novartis: Research Funding; Gilead Sciences: Research Funding; Jazz Pharmaceuticals: Research Funding; Incyte Pharmaceuticals: Research Funding; Karyopharm Therapeutics: Research Funding; Abbvie: Consultancy; Notable Labs: Consultancy. Scott:Bristol Myers Squibb: Consultancy, Honoraria, Other: Advisory Panel, Research Funding; Celgene: Consultancy, Honoraria, Other: Advisor Panel; Alexion: Consultancy; Novartis: Other: Advisory Panel, Research Funding; Jazz Pharmaceuticals: Other: Advisory Panel; Nektar: Other: data and safety monitoring board; Johnson and Johnson: Other: data and safety monitoring board; Incyte: Consultancy. Walter:Janssen Research and Development: Research Funding; Kite Pharma, Inc: Consultancy; Agios: Consultancy, Research Funding; Aptevo Therapeutics: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; Stemline Therapeutics: Research Funding; Amphivena Therapeutics, Inc: Current equity holder in publicly-traded company; Kronos Bio, Inc: Consultancy; Kura Oncology: Consultancy, Research Funding; MacroGenics: Consultancy, Research Funding; Orum Therapeutics, Inc.: Consultancy; Pfizer, Inc: Consultancy, Research Funding; Celgene, Inc: Consultancy, Research Funding; Janssen Global Services, LLC: Consultancy; Selvita: Research Funding; New Link Genetics: Consultancy; Genentech: Consultancy; Bristol Myers Squibb, Inc: Consultancy; ImmunoGen: Research Funding; GSK: Consultancy; Jazz Pharmaceuticals: Consultancy, Research Funding; Boston Biomedical, Inc: Consultancy; BerGenBio, ASA: Consultancy; BioLineRx, LTd: Consultancy, Research Funding; Arog Pharmaceuticals: Research Funding; AbbVie: Consultancy; Race Oncology LTD: Consultancy; Astellas Pharma US, Inc: Consultancy. Marcucci:Novartis: Other: Speaker and advisory scientific board meetings; Agios: Other: Speaker and advisory scientific board meetings; Abbvie: Other: Speaker and advisory scientific board meetings. Becker:Glycomimetics: Research Funding; Accordant Health Services (CVS Caremark): Consultancy; Pfizer Pharmaceuticals: Research Funding; Notable labs: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal